

Affiliations
ABSTRACT
Nutcracker syndrome (NCS) is a rare disease caused by obstruction of renal venous reflux, leading to increased kidney pressure and proteinuria. Diagnosis is based on specific criteria and confirmed through left renal vein colour Doppler ultrasound. Adolescents with a thin body, showing nephrotic syndrome (NS) or proteinuria with glomerular minor lesion (GML) inconsistent with treatment efficacy, should be screened for NCS to prevent misdiagnosis. Herein, we present a rare case of a teenager with NCS and primary NS. This case highlights the importance of considering NCS as a potential underlying condition and emphasises the need for accurate diagnosis to provide appropriate treatment.
Key Words: Nutcracker syndrome, Primary nephrotic syndrome, Glomerular minor lesion.
INTRODUCTION
The left renal vein (LRV) entrapment syndrome, also known as nutcracker syndrome (NCS), is a clinical syndrome characterised by haematuria, proteinuria, left flank pain, and abdominal pain.1 This compression occurs as the LRV passes through the narrow angle between the abdominal aorta (AA) and the superior mesenteric artery (SMA) or the gap between the AA and the spine. In most cases, the angle between the SMA and the AA is too narrow, which is caused by the rapid growth of height during childhood and adolescence, less filling material in the starting part of the SMA, and the drooping of abdominal organs.2
As limited data is available on the prevalence of NCS, diagnosis of NCS is challenging, and a high degree of clinical suspicion is required, as the symptoms can be nonspecific and overlap with other diseases. As a result, NCS is not well-recognised by physicians and can be easily misdiagnosed as glomerulonephritis (GN), IgA nephropathy (IgAN), or urologic stones.3 NCS is commonly combined with chronic nephritis, particularly in cases of IgAN, but cases combined with primary nephrotic syndrome (PNS) are relatively rare. Herein, we present an uncommon case of a teenager with NCS and PNS, who underwent renal biopsy and was diagnosed with glomerular minor lesion (GML).
CASE REPORT
A 15-year boy was admitted due to abnormal proteinuria test for one year and bilateral lower limb oedema for one week. An year ago, his 24-hour urine protein excretion was 4.2 g, and he was diagnosed as PNS. Following the renal biopsy revealing GML, he was treated with prednisone (60 mg/day). However, after two-month therapy, his urinary proteins did not improve significantly, and prednisone was gradually reduced to 30 mg/day. A Chinese herbal medicine was prescribed for him but it did not work. Two months back, the patient started taking tacrolimus (1.5 mg twice a day). One week ago, his urine routine examination revealed protein 4+, occult blood 3+, and uniform-shaped red blood cells. His renal functions were normal and his 24-hour urine protein increased to 8.7 g.
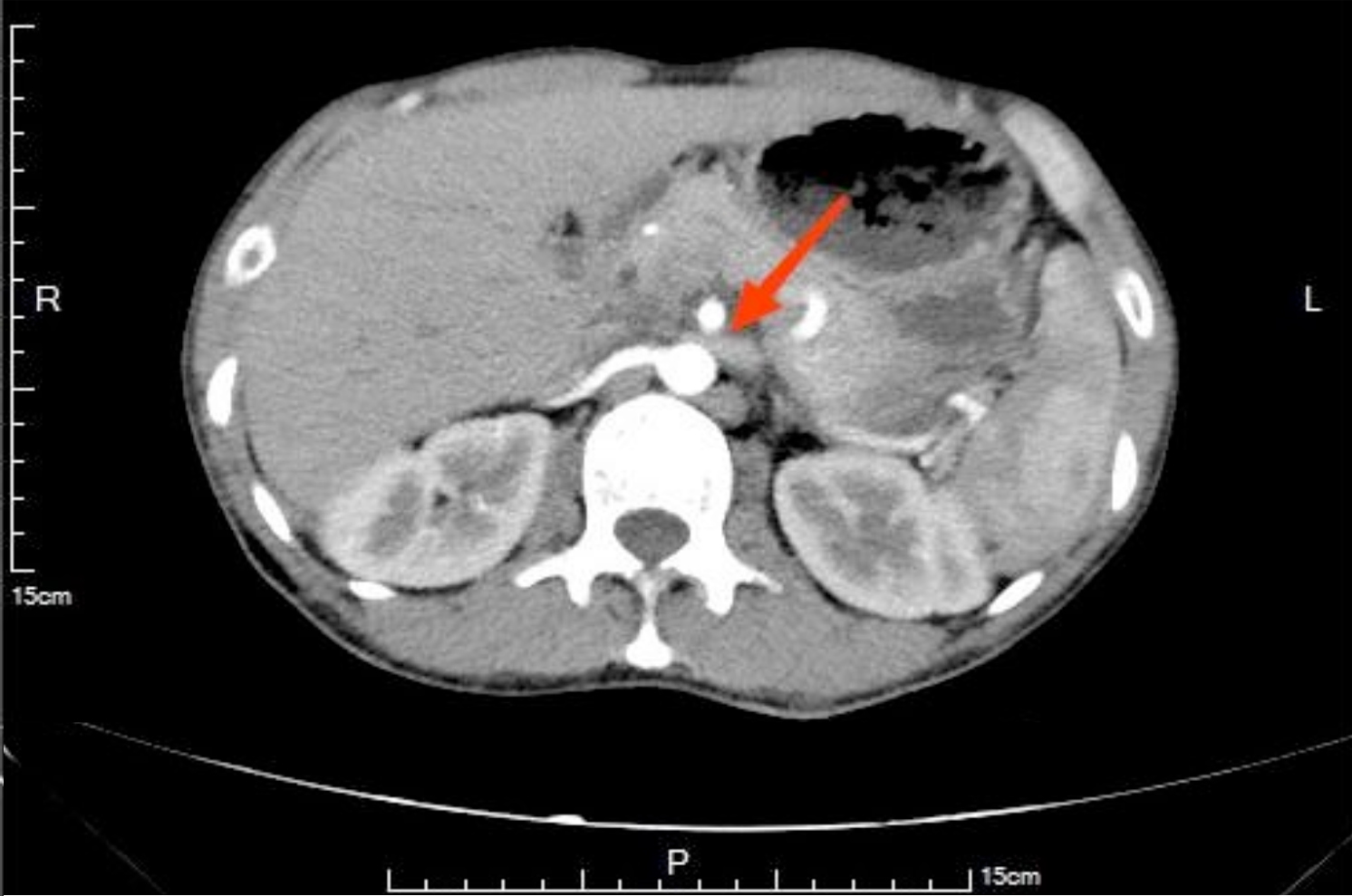 Figure 1: Urogenital contrast-enhanced computed tomography showing the left renal vein significantly compressed at the angle between the abdominal aorta and the superior mesenteric artery (red arrow).
Figure 1: Urogenital contrast-enhanced computed tomography showing the left renal vein significantly compressed at the angle between the abdominal aorta and the superior mesenteric artery (red arrow).
Table I: Laboratory tests done at the hospital.
|
Investigation |
Normal Range |
Day 1 |
Day 14 |
Month 3 |
Month 6 |
|
WBC (×109/L) |
4-10 |
11.3 |
12.3 |
11.6 |
9.2 |
|
Platelets (×109/L) |
100-300 |
345 |
249 |
312 |
273 |
|
Hb (g/L) |
110-150 |
146.3 |
148.1 |
139.8 |
146.7 |
|
Lymphocyte (×109/L) |
1.2-4.8 |
1.9 |
2.1 |
3.4 |
2.9 |
|
ESR (mm/h) |
0-15 |
34 |
/ |
/ |
12 |
|
Alb (g/L) |
40-55 |
25.1 |
24.6 |
37.4 |
45.3 |
|
24hUpro (g) |
0-0.15 |
7.45 |
6.79 |
1.25 |
0.12 |
|
UREA (mmol/L) |
2.60-7.50 |
8.45 |
7.98 |
6.24 |
7.59 |
|
CREA (umol/L) |
41.0-73.0 |
69.4 |
72.1 |
67.5 |
68.2 |
|
CRP (mg/L) |
0-8 |
8.3 |
7.3 |
7.9 |
6.8 |
|
C 3 (g/L) |
0.79-1.52 |
1.25 |
/ |
/ |
/ |
|
C 4 (g/L) |
0.16-0.38 |
0.21 |
/ |
/ |
/ |
|
ASO (IU/mL) |
0-116 |
<25 |
/ |
/ |
/ |
|
RF (IU/mL) |
0-20 |
<20 |
/ |
/ |
/ |
|
AGBM |
- |
- |
/ |
/ |
/ |
|
ANCA |
- |
- |
/ |
/ |
/ |
|
WBC: White blood cell, Hb: Haemoglobin, ESR: Erythrocyte sedimentation rate, Alb: Albumin, 24hUpro: 24 hour urine protein, CREA: Creatinine, CRP: C-reactive protein, Complement 3: C 3, Complement 4: C 4, ASO: Anti streptolysin O, RF: Rheumatoid factor, AGBM: Anti-glomerular basement membrane antibody, ANCA: Anti-neutrophil cytoplasmic antibody. |
|||||
On admission, his general physical examination revealed a tall and thin built with a height of 182 cm and a weight of 61 kg, resulting in a body mass index (BMI) of 18.4 kg/m2. The laboratory examination revealed elevated 24-hour urine proteins (7.45 g) and low serum albumin (25.10 g/L). Doppler ultrasound of his LRV showed a decrease in the angle between the SMA and the AA, causing compression of the LRV at the angle. The diametre of the LRV near the hilum was 4.7 mm (normal: 8-12 mm) in the supine position, and the blood flow velocity at the compressed site was increased to 79 cm/s (normal: 8-15 cm/s). In the standing position, the diametre at the hilum was 12.7 mm (normal: 8-12 mm), and at the compressed site was 3 mm, with a blood flow velocity of 112 cm/s (normal: 8-15 cm/s). Subsequent urogenital contrast-enhanced computed tomography further confirmed the diagnosis (Figure 1). Hence, he was diagnosed with NCS. His treatment was adjusted as prednisone (30 mg once daily) and tacrolimus (1 mg twice daily). Furthermore, the patient was instructed to improve the nutrition and increase the body weight. After three months of the treatment, the patient's weight increased to 69 kg, resulting in a BMI of 21.8 kg/m2. His 24-hour urine proteins was rechecked and showed a significant decrease to 1.25 g, with an increase in serum albumin to 37.40 g/L, indicating the effectiveness of treatment. Regular follow-up visits were scheduled. His urine test was normal, and prednisone and tacrolimus were both discontinued six months later. The main laboratory tests at our hospital are shown in Table I.
DISCUSSION
The currently accepted diagnostic criteria for NCS include several clinical and radiological features.4 According to the existing criteria, a diagnosis of NCS can be made if the following conditions are met: 1) Presence of normal morpho-logy of red blood cells in urine, which is non-glomerular; 2) Urinary protein excretion higher than normal, with 24-hour urinary proteins excretion of <4 mg/kg; 3) Compression and dilation of the LRV visualised on abdominal ultrasound or CT examination; 4) Pressure difference between the LRV and inferior vena cava of >5 cmH2O; 5) Urinary tract bleeding on the left side revealed in cystoscopy examination; 6) Renal biopsy results are normal or show GML; and 7) Other causes of haematuria have been excluded.
This patient had non-glomerular haematuria, and imaging studies revealed compression and dilation of the LRV, which is a typical feature of NCS. His renal biopsy showed GML, which is also consistent with NCS. Furthermore, other possible causes of haematuria were also excluded by the laboratory tests and renal biopsy, supporting the diagnosis of NCS in this case.
Although NCS and proteinuria are related, the amount of proteinuria is usually mild or moderate, and massive proteinuria is extremely rare.5 However, this patient presented with significant proteinuria and hypoalbu-minaemia, which met the clinical diagnostic criteria for NS. The Clinical observations have shown that patients with NCS often exhibit varying degrees of gross haematuria and microscopic haematuria, and misdiagnosis of NCS is common because haamaturia is often mistaken for other diseases such as acute GN, IgAN, urolithiasis.6
This patient was diagnosed with PNS and GML initially. Despite the treatment with sufficient prednisone and immunosuppressive agents, the patient's condition remained unresponsive. Given the patient's tall and thin body built, NCS could not be ruled out, and further diagnosis through LRV colour Doppler ultrasound was necessary. Hence, for patients with the following clinical manifestations, NCS should be ruled out: 1) Adolescents with a BMI <20 kg/m2 and presenting with PNS or proteinuria, with renal biopsy revealing GML, and an inadequate response to treatment, or no renal biopsy; 2) PNS with uniform red blood cells in the urine test; 3) Orthostatic proteinuria, particularly with significant increase in proteinuria and decrease in serum albumin, after excluding diabetic nephropathy; 4) Intermittent gross hematuria with back or abdominal pain, with infection or urolithiasis ruled out; and 5) Significant worsening of proteinuria or haematuria following exercise.
In addition to conservative treatment with nutrition and weight gain, this patient was also treated with prednisone, 30 mg, once daily and tacrolimus, 1 mg, twice daily. Surgical treatment for NCS may involve procedures such as renal vein transposition, renal auto-transplantation, or stenting of the LRV. However, the treatment for NCS should be determined by the specific condition of the patient.7 In the case of underage patients, it is generally recommended to pursue conservative treatment options.8
In conclusion, this report discusses a rare case of PNS combined with NCS as an underlying disease. NCS is a rare syndrome that can easily be misdiagnosed as other diseases. LRV colour Doppler ultrasound is the preferred diagnostic method for NCS. Treatment options include conservative and surgical methods. Adolescents with thin body-built and GML on renal biopsy should be screened for NCS.
PATIENT'S CONSENT:
Written informed consent was obtained from the patient's parent to publish this case report.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
DL: Manuscript writing
XL: Manuscript design and revision.
All authors approved final version of the case report to be published.
REFERENCES